I-Endotoxin Assay Kit ye-Plasma Yomuntu
I-Endotoxin Assay KitngePlasma Yomuntu
1. Ulwazi Lomkhiqizo
I-CFDA isuliweIkhithi yokuhlola yokuhlola i-Endotoxin yomtholampiloihlola izinga le-endotoxin ku-plasma yomuntu.I-Endotoxin iyingxenye enkulu yodonga lwamaseli we-Gram Negative bacteria futhi ingumlamuleli we-microbial obaluleke kakhulu we-sepsis.Amazinga aphezulu e-endotoxin ngokuvamile angabangela ukushisa, izinguquko ekubalweni kwamangqamuzana amhlophe egazi, kwezinye izimo, ukushaqeka kwenhliziyo nemithambo yegazi.Isekelwe ku-factor Cpathway ekuhlolweni kwe-limulus Polyphemus (igazi lenkalankala yehhashi).Nge-kinetic microplate reader nesofthiwe yokuhlola i-endotoxin, ikhithi yokuhlola ye-Endotoxin ithola izinga le-endotoxin ku-plasma yomuntu ngaphansi kwehora elilodwa.Ikhithi iza ne-plasma pre-treatment reagent eqeda izici ezivimbelayo ku-plasma ngesikhathi sokuhlolwa kwe-endotoxin.
2. Ipharamitha yomkhiqizo
Ibanga lokuhlola: 0.01-10 EU/ml
3. Isici somkhiqizo kanye nesicelo
Iza nezixazululo ze-plasma pretreatment, iqeda izici ezivimbelayo ku-plasma yomuntu.
Qaphela:
I-reagent ye-Lyophilized Amebocyte Lysate (LAL) ekhiqizwa yi-Bioendo yenziwe ngegazi elitholakala nge-amebocyte lysate lenkalankala yehhashi.
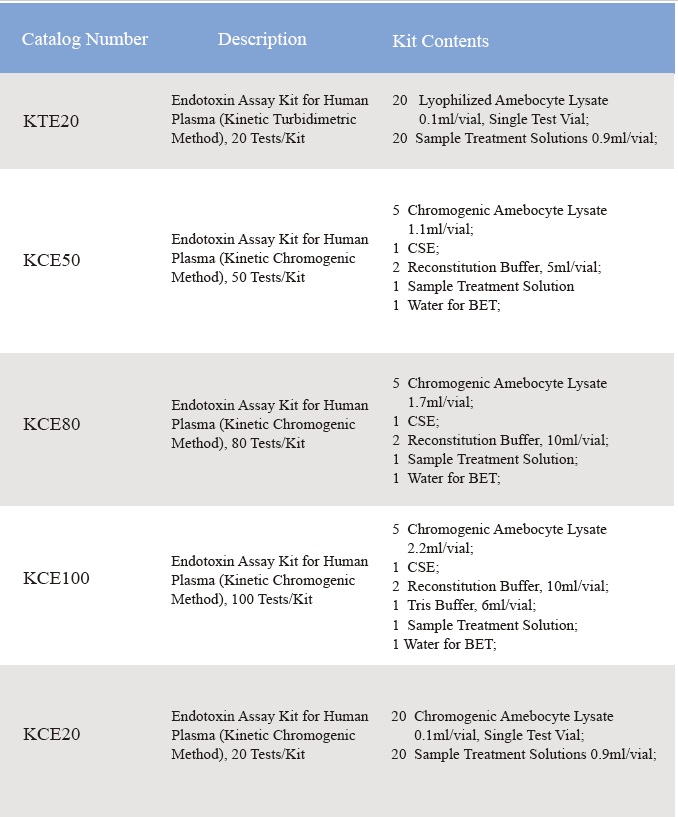
Ukuzwela kwe-Lyophilized Amebocyte Lysate kanye namandla e-Control Standard Endotoxin kuhlolwa ngokumelene ne-USP Reference Standard Endotoxin.I-Lyophilized Amebocyte Lysate reagent kits iza nemiyalelo yomkhiqizo, Isitifiketi Sokuhlaziya, i-MSDS.