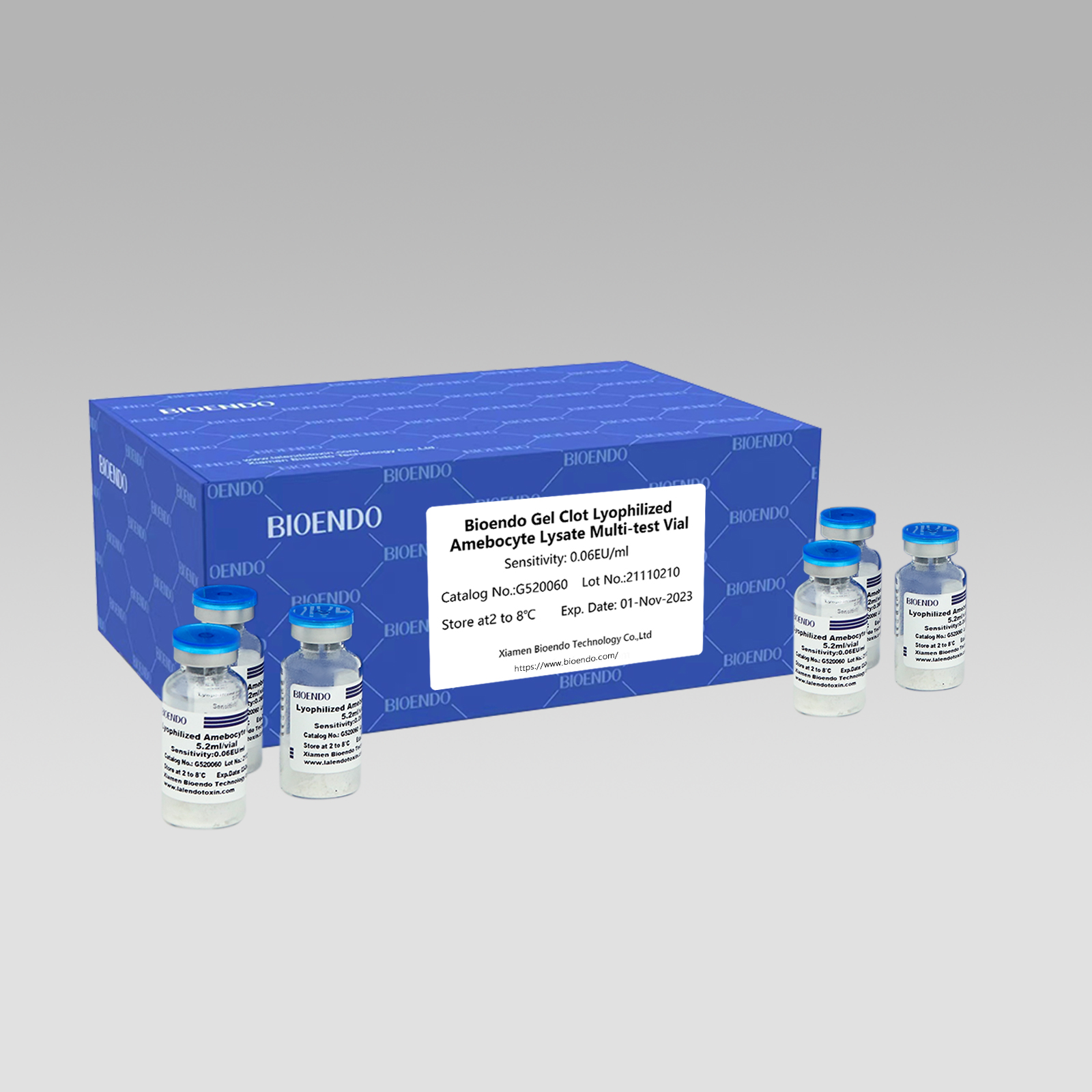
I-Gel Clot Lyophilized Amebocyte Lysate Multi-test Vial G52
Uchungechunge lwe-Bioendo G52 lusetshenziswa kakhulu ekusebenzeni kokuhlolwa kweukuhlolwa kwe-bacterial endotoxinnjengenqubo ye-Bioassay.
1. Ulwazi Lomkhiqizo
Indlela ye-Gel Clot I-Lyophilized Amebocyte Lysate Multi-test Vial iyi-reagent ye-Lyophilized Amebocyte Lysate ekhetha futhi isebenzise indlela yehlule lejeli ukuze ibone i-endotoxin noma i-pyrogen.
Njengendlela esabalele, ukuhlolwa kwe-gel-clot kwe-endotoxin kulula futhi akudingi ithuluzi elithile nelibizayo.I-Bioendo ihlinzeka nge-Gel Clot Lyophilized Amebocyte Lysate - i-LAL reagent ngo-5.2ml ngebhodlela ngayinye.
2. Imingcele yomkhiqizo
Ibanga lokuzwela: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5 EU/ml
3. Isicelo soMkhiqizo
Ukufaneleka komkhiqizo we-endotoxin (pyrogen), amanzi omjovoukuhlolwa kwe-endotoxin, impahla eluhlazaukuhlolwa kwe-endotoxinnoma ukuqapha izinga le-endotoxin phakathi nenqubo yokukhiqiza yezinkampani ezenza imithi noma abakhiqizi bemishini yezokwelapha.
Qaphela:
I-Lyophilized Amebocyte Lysate (i-LAL reagent) ekhiqizwa yi-Bioendo yenziwe nge-lysate ye-amebocytes (amangqamuzana egazi amhlophe) kusuka kunkalankala yehhashi.
Lesi sici esiyingqayizivele sesiyithuluzi elibalulekile embonini yezemithi nemishini yezokwelapha ukuze kutholwe ama-endotoxins amagciwane.Ama-amebocyte enkalankala yehhashi aqukethe into ebizwa nge-Lyophilized Amebocyte Lysate, esabela kuma-endotoxin ebhaktheriya ngokwenza ihlule elifana nejeli.Lokhu kusabela kuyisisekelo sokuhlolwa kwe-LAL, okusetshenziselwa ukuqinisekisa ukuphepha kwemishini yezokwelapha, izidakamizwa, neminye imikhiqizo ehlangana nomzimba womuntu.
Ukusetshenziswa kwe-reagent ye-LAL kuye kwaguqula inqubo yeukutholwa kwe-endotoxinemkhakheni wezokwelapha kunokuhlolwa kweRabit test.Ukuzwela kwayo okungenakuqhathaniswa nokucaciswa kwayo kuyenza ingxenye ebalulekile ekulawuleni ikhwalithi nokuqinisekiswa kokuphepha kwemithi, i-biologics, namadivayisi ezokwelapha.Ukuhlolwa kwe-LAL kuyindlela esheshayo nethembekileukutholwa kwe-endotoxin, ihlinzeka ngemiphumela emizuzwini engama-60.Lokhu kusebenza kahle kuvumela izinqumo ezisheshayo nezinembile mayelana nokukhishwa kwemikhiqizo, ekugcineni kuthuthukiswe ukuphepha okuphelele nokusebenza ngempumelelo kwezokwelapha namadivayisi.
I-Bioendo's Lyophilized Amebocyte Lysate (i-LAL reagent) ikhiqizwa ngaphansi kwamazinga aqinile ekhwalithi ukuze kuqinisekiswe ukusebenza kwayo nokwethembeka.Le nkampani izinikele ekusebenziseni izinqubo ezisimeme ekuvuneni izinkalankala ukuze kuncishiswe noma yimuphi umthelela omubi kubantu bazo.Ngokubeka phambili inhlalakahle yalezi zidalwa, i-Bioendo iqinisekisa ukuhlinzekwa okuqhubekayo kwalesi sisetshenziswa esibalulekile sokukhiqiza ama-reagents e-LAL.Ukwengeza, imizamo yocwaningo nentuthuko eqhubekayo igxile ekuthuthukiseni ukusebenza kanye nokuguquguquka kweI-LAL ihlola i-endotoxin, baqhubekisela phambili ukusetshenziswa kwabo embonini yezokwelapha neyemithi.
Indlela ye-gel clotIsivivinyo se-LAL, i-lysate reagent eyenziwe kabusha ithola okungenani izivivinyo ezingama-50 ngevial ngayinye:
| Inombolo Yekhathalogi | Ukuzwela (EU/ml noma i-IU/ml) | ml/ibhodlela | Izivivinyo/Isitsha | Izitsha/Iphakethe |
| I-G520030 | 0.03 | 5.2 | 50 | 10 |
| I-G520060 | 0.06 | 5.2 | 50 | 10 |
| G520125 | 0.125 | 5.2 | 50 | 10 |
| I-G520250 | 0.25 | 5.2 | 50 | 10 |
| I-G520500 | 0.5 | 5.2 | 50 | 10 |
Isimo somkhiqizo:
I-Lyophilized Amebocyte Lysate - ukuzwela kwe-reagent ye-LAL kanye namandla okulawula e-Endotoxin Ejwayelekile ayahlolwa ngokumelene ne-USP Reference Standard Endotoxin.Ikhithi ye-Lyophilized Amebocyte reagent iza nemiyalelo yomkhiqizo, Isitifiketi Sokuhlaziya, i-MSDS.
Uyini umehluko phakathi kwe-bioendo single test vial kanye ne-multiple test vial?
● Ukuhlolwa okukodwa: hlanganisa kabusha eyodwaukuhlolwa kwe-limulus lysatenoma ebizwalimulus amebocytengamanzi e-BET ku-vial yengilazi noma i-ampoule yengilazi.
● Ukuhlola okuningi: hlanganisa kabusha i-lysate reagent ngamanzi e-BET, bese wengeza inani elimakiwe le-lysate reagent elandela i-COA kushubhu yokusabela noma ipuleti lomthombo ozolisebenzisa.Awukho umehluko kunqubo yesampula yokucubungula ngaphambilini;ngokuya ngenani lokuhlola elisetshenzisiwe, usayizi wesampula osetshenziselwa ukuhlolwa okukodwa mkhulu kunosayizi wesampula osetshenziselwa ukuhlola okuningi.
Kungani ikhithi ye-gel clot assay i-G52 ikhethekile ngenani lamasampula amaningi?
1. I-Reagent ye-LAL yokuhlola eminingi yokutholwa kwe-endotoxin ekusetshenzisweni kwezinqubo zokuhlola ze-LAL zamasampuli amaningi.
2. Uchungechunge lwe-G52 lwe-Gel clot endotoxin assay multi test vial ayidingi isifundi se-microplate esiyinkimbinkimbi.Esivivinyweni se-LAL inqubo yayo yokufukamela ngokugeza ngamanzi noma nge-incubator yokushisa eyomile iyithuluzi elilula.
3. Ikhwalithi ephezulu yokugcina ye-endotoxin free tube (<0.005EU/ml) kanye nekhwalithi ephezulu yamathiphu angenayo i-pyrogen (<0.005EU/ml) njengento esebenzisekayo eqinisekisiwe ukuze kuqinisekiswe umphumela olungile.
4. Ukukhetha ibhodlela yokuhlola ye-Bioendo eyodwa ye-LAL noma ibhodlela yokuhlola eminingi ye-LAL ngenani lamasampula, okuhlosiweUkuhlolwa kwe-LAL kwama-pyrogensukutholwa.
Imikhiqizo ehlobene ekuhlolweni kokuhlolwa kwe-endotoxin:
Amanzi Okuhlola I-Bacterial Endotoxins Test (BET), Ncoma i-TRW50 noma i-TRW100
Ishubhu yengilazi yamahhala ye-Endotoxin (i-dilution tube), Ncoma i-T1310018 ne-T107540
Amathiphu wamahhala we-Pyrogen, Ncoma i-PT25096 noma i-PT100096
Pipettor, Ncoma i-PSB0220
I-Test Tube Rack
I-Incubation Instrument (Ukugeza Amanzi noma I-Dry Heat Incubator ), ukuncoma i-Bioendo Dry Heat Incubator TAL-M2 iyizimbobo ezingu-60 i-modular eyodwa.
I-Vortex Mixter, Ncoma i-VXH.
Lawula I-Endotoxin Ejwayelekile, i-CSE10V.